Environmental standards
Marine finfish farming across Scotland operates using open-net pens. Several hundred thousand fish will be farmed at most farms. Fish excreta; uneaten food; used fish medicines and other chemicals are discharged through the nets into the marine environment. Sea lice larvae hatching from adult female sea lice on the farmed fish can also pass through the nets into the surrounding coastal waters.
Discharges
The heavier, organic particles (fish faeces and uneaten food), and any medicines sticking to them, are deposited on the sea floor. Natural biological processes then break down and assimilate the organic wastes over time. Dissolved chemicals released from the farm, including some medicine residues and compounds of nitrogen, together with chemicals that are re-suspended from the deposited wastes, disperse in the sea until broken down or assimilated.
To protect the marine environment, the quantity of wastes released from finfish farms must be appropriately matched to the sea’s capacity to disperse and assimilate them so that they do not reach levels that would harm sea life. It is SEPA’s role as environmental regulator to make sure this is the case.
The two main factors that determine whether wastes from a fish farm are sufficiently diluted are:
- The quantity of the wastes being released. This depends on the size of the farm
- The capacity of the sea to disperse the wastes. This varies according to strength of tides and, to a lesser extent, winds
The quantities of medicine released depend in part on farm size but also on a site’s vulnerability to diseases and infections and the effectiveness non-chemical disease management techniques used by the farm.
Farm operators are required to monitor the seabed around the farm to ensure appropriate environmental standards are met. The monitoring requirements are outlined in individual farm permits and associated environmental monitoring plans, as well as our published performance standards. All existing farms are being transitioned onto our latest permit template during 2024. This will standardise monitoring requirements across all farms. While that transition is underway, we have adopted a Temporary Regulatory Position on sampling on sampling to ensure consistency.
Sea lice larvae
Sea lice larvae can infect juvenile salmon (post-smolts) as they swim through coastal waters in April and May on their way to their feeding grounds in the North Atlantic. Infections with only a few sea lice can result in harm and increased mortality. To protect wild salmon from harm from sea lice from fish farms, the concentrations of infective-stage larvae produced by farms need to be kept below concentrations likely to result in harmful levels of infection.
We have established a series of wild salmon protection zones along the West Coast and Western Isles. These are sea lochs and other bottlenecks through which salmon populations must migrate to reach more open sea. We use a sea lice exposure threshold in these zones for assessing their capacity to accommodate sea lice larvae emanating from farms without a significant risk to wild salmon post-smolts.
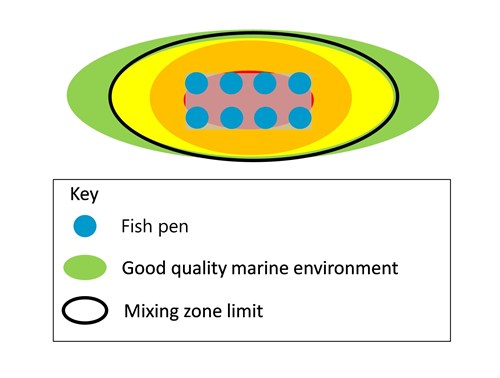
Mixing zone extent
Immediately around fish farm pens, there is typically a zone in which organic waste such as fish excreta and uneaten food are not fully mixed and dispersed in the surrounding sea. As this material falls onto the seabed it can smother animals, particularly those that live fixed in one place. The breakdown of this material then lowers oxygen levels and releases chemicals, such as ammonia, that are toxic to marine organisms.
Some medicines are incorporated into fish food (“in-feed medicines”). This medicated food is fed to the fish over a number of days and residues of the medicines are excreted by the treated fish. The medicine residues in the excreta and any uneaten medicated food sink to the seabed alongside other organic waste. These residues can then accumulate on the seafloor to levels that are toxic to seabed organisms.
Our regulatory framework limits the maximum area of the mixing zone (Figure 1). The limit is equivalent to the area lying within 100 metres of the pens in all directions. Wastes are rarely deposited on the seabed in a perfectly symmetrical pattern around farms. Tides and seabed topography tend to spread material further in some directions than others, typically producing something resembling an ellipse-like shape of deposited material. Accordingly, the framework provides for mixing zones that are not symmetrical. Mixing zones may extend more than 100 metres from the pens in some directions provided their maximum area does not exceed the area that a symmetrical, 100 metre mixing zone would have.
Seabed mixing zone limit - compliance assessment method
The seas around Scotland support a wide variety of plant and animal life. This includes species and habitats that are particularly rare or threatened, many which are protected under law. Mixing zones in the wrong location could adversely affect these and also affect other people’s uses of the sea, such as for shellfish farming. For all proposed fish farm developments, we ensure that risks to protected wildlife and to other uses of the sea are properly assessed. We do not permit mixing zones that would extend into locations where they would be likely to compromise the conservation status of marine protected areas [1] or priority marine features [2]; or other people’s uses of the sea.
Figure 1. The mixing zone
Quality standards
Our regulatory framework requires farm operators to manage their sites so that there is no significant adverse impact on the biodiversity of seabed life beyond the edge of the permitted mixing zone. To make sure this is the case, we use the results of sophisticated computer modelling to assess whether proposed farm developments will be able to operate without compromising biological and chemical environmental standards for good status beyond the mixing zone. When making these assessments, we will consider the risks posed in combination with any existing pressures on environmental quality from other activities. For operational farms, we use the results of environmental monitoring to check that good quality is being maintained.
The environmental standards required for good quality are:
- standards for good status laid down in law in the form of directions issued by Scottish Ministers
or
- where an appropriate standard has not yet been specified in Directions, a standard representing equivalently good quality that we have derived using the best available science, including published research and expert advice.
The Directions do not currently include quality standards for seabed habitats dominated by large stones, rock or other hard materials. We will use visual imagery survey results to assess the condition of these habitats where they lie within the mixing zone of a farm.
Within the mixing zone, operators must ensure that wastes do not accumulate to levels that would compromise the biological process needed to breakdown and assimilate them. We use the latest computer modelling to assess whether proposed developments will be able to operate without compromising these processes. Environmental monitoring results are used to check that the required standards are being maintained at operational sites.
The most common environmental standards that are used under our regulatory framework to ensure the protection of the seabed are listed in Table 1. We will refuse to grant an authorisation for proposed farm developments that are unlikely, to achieve seabed quality standards.
Table 1: List of common standards for protecting the seabed
Number of species, and abundance, of the re-worker polychaete worms:
- all polychaete species listed as “AMBI Group V” 1 species
- Ophryotrocha species; and
- Boudemos species
| What the standard applies to | Where the standard applies | The type of standard | How the standard is measured | What the standard is |
|---|---|---|---|---|
| Condition of invertebrate animals living in soft sediments | At mixing zone limit & beyond | Good status standard | Infaunal quality index method as specified under 2014 Standards Directions | 0.64 as minimum value at any time |
| Most extreme permitted effect of waste deposition on sea bed invertebrate animal communities | In mixing zone | Basic seabed functioning standard | A minimum of 2 species with a combined abundance of more than 1,000 individuals per m-2 | |
| Maximum concentration of in-feed sea lice medicine, emamectin benzoate | At mixing zone limit & beyond | Good status standard | ng per kg of marine sediment (dry weight) | 272 |
Note 1. “AMBI Group V” species as listed in Borja, A., Franco, J., Perez, V. (2000). A Marine Biotic Index to Establish the Ecological Quality of Soft-Bottom Benthos Within European Estuarine and Coastal Environments. Marine Pollution Bulletin 40: 1100-1114
Medicine residues from topical treatments
Some sea lice medicines are applied by immersing the fish in a solution of the medicine topical medicines). This may be done by creating a bath on the farm or by taking the fish to a container of medicine located on a boat, called a well-boat. Once the fish have been treated, the water containing the medicine residues is released into the sea where it disperses away from the farm.
We regulate discharges of medicine residues from bath treatments. Under our regulatory framework, fish farm operators have to ensure that within specified periods of time after a medicine’s discharge from a bath into the sea, it has been sufficiently diluted to achieve the environmental standards defined for each of those periods of exposure to the medicine (Table 2). The periods of time vary depending on the medicine used. The two medicines currently used for bath treatments are the organophosphate, azamethiphos, and the pyrethroid, deltamethrin.
We use the results of modelling to assess whether the environmental standards will be met. We normally require marine modelling to allow us to assess whether the farm will be able to meet the required standards. It also allows us to assess where the plume from the discharge is likely to travel as it disperses and assimilates into the surrounding sea. We will refuse to grant an authorisation for proposed discharges of bath medicines where:
- the environmental standards would not be met; or
- an insufficiently diluted plume is likely to interact with, and pose a risk to the conservation status of, protected species or habitats; or adversely affect the interests of other users of the marine environment
Discharges of treatment solutions from well-boats are currently regulated separately by the Marine Directorate. The Scottish Government is preparing to transfer responsibility for the control of these discharges to SEPA.
Table 2: Environmental standards for topical medicines
| Medicine | Maximum allowable concentration (ng per litre) | Annual average (ng per litre) | |||||
|---|---|---|---|---|---|---|---|
| 3 hours after discharge | 6 hours after discharge | 12 hours after discharge | 24 hours after discharge | 48 hours after discharge | 72 hours after discharge | ||
| Azamethiphos | 250 | n/a | n/a | 150 | n/a | 40 | |
| Deltamethrin | 9 | 6 | 4 | 2 | 1 | n/a | |
| Cypermethrin | 0.06 | 0.008 | |||||
As part of our Sector Plan for Finfish Aquaculture, we will be reviewing our approach to how we protect the marine environment from discharges of topical medicines.
Compounds of nitrogen
Fish farms release compounds of nitrogen, principally ammonia and urea, into sea water. The vast majority of this enters from fish excreta. Dissolved organic compounds of nitrogen are also released and re-suspended into the water from organic waste deposited on the seabed. Some of these nitrogen compounds have the potential to be toxic if they are present in sufficiently high concentrations. Dissolved nitrogen compounds are also taken up as a nutrient by phytoplankton, seaweeds and other marine plants. In excess, dissolved nitrogen can accelerate growth and result in a cascade of changes to the biodiversity and functioning of marine ecosystems.
Under our regulatory framework, we use guidelines prepared by the Marine Directorate to screen proposals to assess the likelihood of risks to the marine environment from compounds of nitrogen. Where screening indicates there may be little or no capacity for further inputs of nitrogen into a sea area, fish farm businesses wishing to develop or expand farms have to demonstrate, including by means of suitable marine modelling, that their proposals will not lead to adverse impacts.
As part of our Sector Plan for Finfish Aquaculture, we will be reviewing whether we need to strengthen our approach to protecting the marine environment from discharges of nutrients.
Medicines and other chemical treatments for farmed fish can be extremely toxic to sealife at very low concentrations. The discharge of any medicines and other chemicals that are likely to be toxic to marine life requires prior-authorisation under our regulatory framework. This includes proposals to discharge medicines and other chemicals not currently authorised at any farm sites, for example, newly developed medicines and medicines that stopped being used some time ago but which the sector is seeking to use again.
Before considering authorising proposed discharges of new medicines and other chemicals not currently authorised under the framework, we will require sufficient data and time in advance:
- to allow an assessment of the likely risk to marine life; and
- where there is such a risk, for a scientifically robust environmental standard to be derived
In the absence of the above, we will refuse to grant an authorisation to discharge medicines and/or other chemicals which are not currently authorised under the regulatory framework.
The way we protect marine life from discharges relies on the sea’s capacity to disperse, break down and assimilate any substances in those discharges that have potential to cause harm if they are present at sufficiently high concentrations. We use environmental standards to limit concentrations in the environment to levels that do not cause harm whilst breakdown and assimilation take place.
Some potential medicines and other chemicals not currently authorised under the framework may be more persistent in the environment or more likely to bio-accumulate than the currently authorised range of medicines. The risk to marine life from such medicines and other chemicals cannot be effectively controlled by relying on the sea’s capacity to disperse, breakdown and assimilate substances. This is because their persistence means that they can accumulate in the environment overtime to harmful levels. For medicines and other chemicals with such properties, we will expect farms to capture and de-nature residues prior to any discharge being authorised.
Sea lice interaction with salmon
The salmon louse (Lepeophteirus salmonis) is a parasite of salmon and sea trout. During their early post-hatching larval stages, salmon lice are free-living and non-feeding in the sea. Once they have moulted into the infective, copepodid stage, if they encounter a salmon or sea trout, they can attach to the surface of the fish’s skin where they can feed and mature into adults. The lesions they cause on the skin of the host fish cause osmoregulatory stress and can lead to secondary infections and death. The number of sea lice a salmon post-smolt can tolerate without suffering significant harm depends on its size. The smaller the fish, the fewer sea lice it can tolerate.
Farmed salmon and rainbow trout act as hosts for sea lice, and, because of the large number of fish they can hold, fish farms can contribute significantly to local concentrations of infective-stage salmon lice in the sea.
The number of sea lice with which a wild salmon post-smolt may become infected depends on the concentration of infective-stage sea lice in the sea through which it swims; and the time it is exposed to those concentrations. Exposure to a high concentration of infective-stage sea lice for an extended period would lead to infestation with more lice than exposure to the same concentration for a short time.
Sea lice exposure threshold
Our sea lice regulatory framework uses a sea lice exposure threshold to help manage interactions between sea lice from fish farms and wild Atlantic salmon post-smolts.
The exposure threshold is expressed in sea lice per m2 days and describes the maximum cumulative concentration of infective-stage sea lice (integrated over the upper 2 metres of sea) to which salmon post-smolts can be exposed before they would be likely to be infected with a harmful number of sea lice.
Sea lice exposure threshold
The exposure to infective-stage sea lice per m2 days of the 95th percentile of all virtual salmon post-smolt exposures = 0.7
Notes
“Sea lice per m2 days” means the average concentration of infective-stage sea lice a salmon post-smolt experiences during its passage through a wild salmon protection zone multiplied by the duration of that passage in days (i.e., For a passage time of 24 hours, exposure to an average concentration of 0.7 sea lice per m2 would be equivalent to the exposure threshold; for a passage time of 12 hours, exposure to an average concentration of 1.4 sea lice per m2 would be equivalent to the exposure threshold; and for a passage time of 48 hours, exposure to an average concentration of 0.35 sea lice per m2 would be equivalent to the exposure threshold
The 95th percentile exposure value is rounded to one decimal place for the purpose of applying the threshold
To apply the threshold, the potential exposures of salmon post-smolts to infective-stage sea lice are simulated by modelling the emigration of large numbers of “virtual” salmon post-smolts from each salmon river mouth through the corresponding Wild Salmon Protection Zones to the open sea over different time steps from 1st April to 31st May. For each virtual salmon post-smolt, the modelled cumulative exposure to infective-stage sea lice is calculated. This is done by identifying the model grid cells through which the virtual smolt passes and adding up the product of the instantaneous concentration of the corresponding grid cell by the time spent in that grid cell (based on a uniform fish swim speed of 12.5 cm/s). The 95th percentile of all exposures within a given Wild Salmon Protection Zone is then compared against the sea lice exposure threshold.
The sea lice exposure threshold has been derived from the findings of two different and independent research approaches, one led by Norwegian scientists and the other led by Scottish scientists.
